Glycobiology
Background
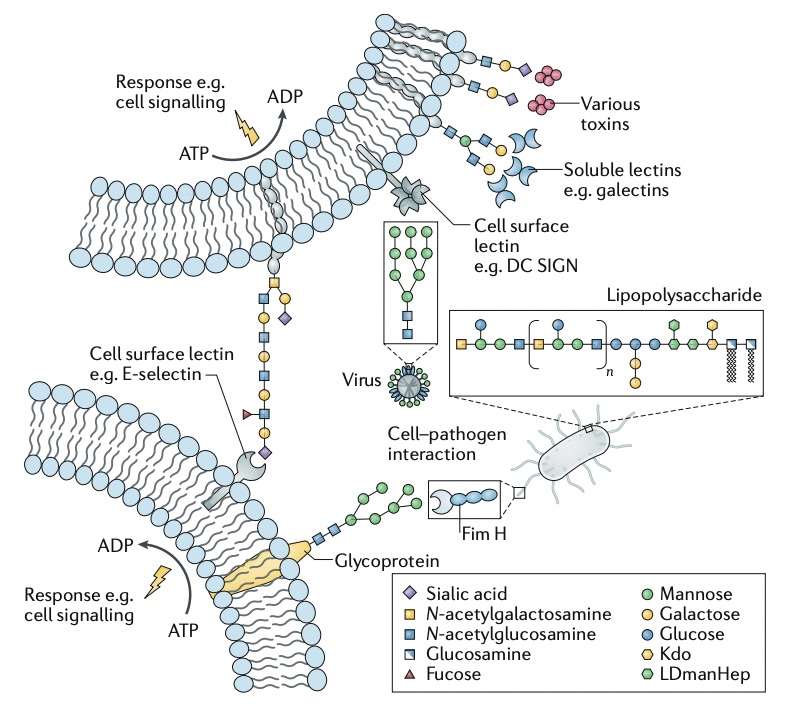
Glycobiology, also known as glycan, is the study of the structure, biosynthesis, biology, and evolution of saccharides that are widely distributed in nature in all living forms. Glycobiology is a rapidly expanding field of study that is critical for both cellular biology and studies of carbohydrate-based drugs and therapeutics:
- Glycans play a role in communication between cells and their external environment.
- Glycosylation is important for protein folding and quality control and defines the adhesive properties of proteins and cells.
- Glycans play an important role in the development of multicellular organisms and their functions are determined by their unique structures.
- Glycosylation is an important determinant of the efficacy of biologics such as therapeutic antibodies.
Research Area of Glycobiology in Different Species
- Yeasts
As biopharmaceuticals with humanized N-linked oligosaccharides, the suppression of yeast-specific O-mannosylation is important to reduce immune response and to improve heterologous protein productivity in the production of biopharmaceuticals. Present study demonstrated that Saccharomyces cerevisiae strain is capable of producing a glycoprotein with humanized Man5GlcNAc2 N-linked oligosaccharides, an intermediate of mammalian hybrid- and complex-type oligosaccharides while suppressing O-mannosylation. This strain was generated by firstly introducing msdS encoding α-1,2-mannosidase into a strain synthesizing Man8GlcNAc2 N-linked oligosaccharides, then disrupting PMT1 and PMT2, using a mutagenesis technique that is based on the disparity theory of evolution and finally disrupting vacuolar proteases PEP4 and PRB1.
- Rice
Although the differences in protein glycosylation between sexes have already been observed in the rice pest insect Nilaparvata lugens (N. lugens), the functionality of differential N-glycosylation between sexes is yet unknown. Recent study indicated that comparison of N-glycopeptides sites from the adult stages of N. lugens revealed striking differences in protein N-glycosylation between sexes. Additionally, differential glycan composition between males and females was observed for proteins shared across sexes.
- Arabidopsis thaliana (Arabidopsis)
N-glycosylation plays multiple roles in regulating stress tolerance of plants. Study has shown that an obvious decrease in photosynthetic capacity and dry mass were detected in alg3-3 and cgl1-1, two typical mutants in N-glycosylation process of Arabidopsis. The maximal photochemical efficiency of PSII, which reflected the photochemical of plant, decreased significantly in cgl1-1. Also, a similar tendency was observed in alg3-3. Besides, N-glycosylation was also required to maintain the stability of a chloroplast-located protein CAH1, which was closely related to photosynthesis.
Reference
- Chiang, A. W. T., et al. "Systems glycobiology for discovering drug targets, biomarkers, and rational designs for glyco-immunotherapy." Journal of biomedical science 28.1 (2021): 50 Distributed under Open Access license CC BY 4.0, without modification.
Our provided featured target antibody products including but not limited to:
AibGenesis™ Mouse Anti-AGL1 Antibody (CBMOAB-00431HCB) (CAT#: CBMOAB-00431HCB)
-
- Host species: Mouse
- Species Reactivity: C. elegans (Caenorhabditis elegans), A. thaliana (Arabidopsis thaliana)
- Application: WB, ELISA
- Protein: 4-alpha-glucanotransferase
- Size: 0.5mg, 1mg, 200µg
- Conjugate: AP, APC, Biotin, Consult us more, Cy3, Cy5, Cy5.5, Cy7, FITC, HRP, NONE, PE, PerCP
- Alternative Names: AGL (Amylo-1,6-GLucosidase, 4-alpha-glucanotransferase) glycogen debranching enzyme, agl-1
AibGenesis™ Mouse Anti-GALT1 Antibody (CBMOAB-04249HCB) (CAT#: CBMOAB-04249HCB)
-
- Host species: Mouse
- Species Reactivity: C. elegans (Caenorhabditis elegans), A. thaliana (Arabidopsis thaliana), Fruit fly (Drosophila melanogaster)
- Application: WB, ELISA
- Protein: Beta-1,4-galactosyltransferase galt-1
- Size: 0.5mg, 1mg, 200µg
- Conjugate: AP, APC, Biotin, Consult us more, Cy3, Cy5, Cy5.5, Cy7, FITC, HRP, NONE, PE, PerCP
- Alternative Names: Protein GALT-1, galt-1
AibGenesis™ mouse Anti-Rubisco Antibody (MOFAB-030W) (CAT#: MOFAB-030W)
-
- Host species: mouse
- Species Reactivity: Rice, Cucumber (Cucumis sativus), Plant, Plants
- Application: IA
- Protein: Ribulose-1, 5-bisphosphate carboxylase/oxygenase
- Size: NONE
- Conjugate: None
- Alternative Names: Ribulose-1, 5-bisphosphate carboxylase/oxygenase, Rubisco
Rabbit Anti-Gliadin Antibody (MOF032922W82) (CAT#: MOF032922W82)
-
- Host species: Rabbit
- Species Reactivity: Wheat gliadin
- Application: ELISA, IHC-P, IHC-Fr, ICC, IF
- Protein: ergot protein
- Size:
- Conjugate:
- Alternative Names: Celiac disease, gliadin - wheat
AibGenesis™ Mouse Anti-B6.1 Antibody (MOFY-0522-FY20) (CAT#: MOFY-0522-FY20)
-
- Host species: Mouse
- Species Reactivity: Chicken
- Application: FC, IHC, IP
- Protein: LOC396098
- Size: 0.5mg
- Conjugate: Biotin, FITC, NONE, PE
- Alternative Names: B6.2, Bu-1, Bu-1b
AibGenesis™ Mouse Anti-SIRPA Antibody (MOFY-0522-FY60) (CAT#: MOFY-0522-FY60)
-
- Host species: Mouse
- Species Reactivity: Porcine, Cattle (Bos taurus), Chimpanzee (Pan troglodytes), Horse (Equus caballus), Marmoset
- Application: FC
- Protein: Signal Regulatory Protein Alpha
- Size: 100µg
- Conjugate: Biotin, FITC, NONE, PE
- Alternative Names: Signal Regulatory Protein Alpha, CD172 Antigen-Like Family Member A, Inhibitory Receptor SHPS-1, Macrophage Fusion Receptor, PTPNS1, SHPS1, SIRP, P84, BIT, MFR, Brain-Immunoglobulin-Like Molecule With Tyrosine-Based Activation Motifs, Brain Ig-Like Molecule With Tyrosine-Based Activation Motifs, Protein Tyrosine Phosphatase, Non-Receptor Type Substrate 1, Tyrosine-Protein Phosphatase Non-Receptor Type Substrate 1, Tyrosine Phosphatase SHP Substrate 1, Signal-Regulatory Protein Alpha-1
AibGenesis™ Mouse Anti-FBN1 Antibody (MOFY-0522-FY82) (CAT#: MOFY-0522-FY82)
-
- Host species: Mouse
- Species Reactivity: Human, Bovine, Japanese, C. elegans (Caenorhabditis elegans), Cattle (Bos taurus), Chicken (Gallus gallus), Dog (Canis lupus familiaris), Marmoset, O. mykiss (Oncorhynchus mykiss), Pig (Sus scrofa), Rhesus (Macaca mulatta), Zebrafish (Danio rerio)
- Application: IHC, EM, WB, IP
- Protein: Fibrillin 1
- Size: 200µg
- Conjugate: Biotin, FITC, NONE
- Alternative Names: Fibrillin 1, Asprosin, FBN, Fibrillin 1 (Marfan Syndrome), Fibrillin-1 Preproprotein, Marfan Syndrome, Fibrillin 15, Fibrillin-1, GPHYSD2, ACMICD
AibGenesis™ Mouse Anti-BLV gp51-G Antibody (MOFY-0522-FY203) (CAT#: MOFY-0522-FY203)
-
- Host species: Mouse
- Species Reactivity: Bovine
- Application: RIPA, FC, ELISA
- Protein: Bovine Leukemia Virus (BLV) MAb gp51-G
- Size:
- Conjugate:
- Alternative Names: BLV gp51-G, Bovine Leukemia Virus (BLV) MAb gp51-G
AibGenesis™ Mouse Anti-BLV gp51-G D-D Antibody (MOFY-0522-FY204) (CAT#: MOFY-0522-FY204)
-
- Host species: Mouse
- Species Reactivity: Bovine
- Application: RIPA, FC, WB
- Protein: Bovine Leukemia Virus (BLV) MAb gp51 D-D'
- Size:
- Conjugate:
- Alternative Names: BLV gp51-G D-D', Bovine Leukemia Virus (BLV) MAb gp51 D-D'
Rabbit Anti-FGB Antibody (MOFY-0722-FY268) (CAT#: MOFY-0722-FY268)
-
- Host species: Rabbit
- Species Reactivity: Pig, Cat (Felis catus), Cattle (Bos taurus), Chicken (Gallus gallus), Dog (Canis lupus familiaris), Donkey (Equus asinus), Frog (Xenopus laevis), Goat (Capra hircus), Horse (Equus caballus), Mallard (Anas platyrhynchos), Pig (Sus scrofa), Rabbit (Oryctolagus cuniculus), Rhesus (Macaca mulatta), Sheep (Ovis aries), Zebrafish (Danio rerio)
- Application: WB, IHC, ICC, IP
- Protein: Fibrinogen Beta Chain
- Size: 100µL
- Conjugate: None
- Alternative Names: Fibrinogen Beta Chain, Fibrinogen, B Beta Polypeptide, Epididymis Secretory Sperm Binding Protein Li 78p, Beta-Fibrinogen, HEL-S-78p
Rabbit Anti-TG Antibody (MOFY-0722-FY317) (CAT#: MOFY-0722-FY317)
-
- Host species: Rabbit
- Species Reactivity: Bovine, Cattle (Bos taurus), Dog (Canis lupus familiaris), Fruit fly (Drosophila melanogaster), Goat (Capra hircus), Pig (Sus scrofa), Rat (Rattus norvegicus), Zebrafish (Danio rerio)
- Application: WB, IHC, ICC, IP
- Protein: TG Gene(Protein Coding) Thyroglobulin
- Size: 100µL
- Conjugate: None
- Alternative Names: Thyroglobulin, AITD3, TGN, Tg
Rabbit Anti-FN1 Antibody (MOFY-0722-FY423) (CAT#: MOFY-0722-FY423)
-
- Host species: Rabbit
- Species Reactivity: Goat, Cattle (Bos taurus), Chicken, Chicken (Gallus gallus), Chimpanzee (Pan troglodytes), Dog (Canis lupus familiaris), Frog (Xenopus laevis), Horse (Equus caballus), Marmoset, Medaka (Oryzias latipes), Rabbit (Oryctolagus cuniculus), Rhesus (Macaca mulatta)
- Application: WB, IHC, ICC, IP
- Protein: Fibronectin 1
- Size: 100µL
- Conjugate: None
- Alternative Names: Fibronectin 1, Cold-Insoluble Globulin, Migration-Stimulating Factor, CIG, FN, Fibronectin, GFND2, SMDCF
AibGenesis™ Mouse Anti-Endoglycosidase [EndoS] (AA 1-243) Antibody, IgG1 kappa (MOFY-0922-FY396) (CAT#: MOFY-0922-FY396)
-
- Product Overview: MOFY-0922-FY396 is a mouse antibody against Endoglycosidase [EndoS]. It can be used for Endoglycosidase [EndoS] detection in Western Blot, Immunoprecipitation, Immunofluorescence, ELISA.
- Host species: Mouse
- Species Reactivity: S. equi subsp. equi 4047
- Application: WB, IP, IF, ELISA
- Protein: Endoglycosidase [EndoS]
- Size:
- Alternative Names: Endoglycosidase [EndoS]
AibGenesis™ Mouse Anti-OVA Antibody, IgG1 (MOFY-0922-FY406) (CAT#: MOFY-0922-FY406)
-
- Host species: Mouse
- Species Reactivity: Chicken, Fruit fly (Drosophila melanogaster)
- Application: IP, ELISA
- Protein: Ovalbumin
- Size: 200µg
- Alternative Names: OVA, Ovalbumin
AibGenesis™ Mouse Anti-OVA Antibody, IgG1 (MOFY-0922-FY409) (CAT#: MOFY-0922-FY409)
-
- Host species: Mouse
- Species Reactivity: Chicken, Fruit fly (Drosophila melanogaster)
- Application: WB, ELISA
- Protein: Ovalbumin
- Size: 200µg
- Alternative Names: OVA, Ovalbumin
AibGenesis™ Mouse Anti-OVA Antibody, IgG1 (MOFY-0922-FY410) (CAT#: MOFY-0922-FY410)
-
- Host species: Mouse
- Species Reactivity: Chicken, Fruit fly (Drosophila melanogaster)
- Application: IP, ELISA
- Protein: Ovalbumin
- Size: 200µg
- Alternative Names: OVA, Ovalbumin
To download a Certificate of Analysis, please enter a lot number in the search box below. Note: Certificate of Analysis not available for kit components.
Lot Number