To date, monoclonal antibody technology is still the standard method to generate monoclonal antibodies (mAbs). Creative Biolabs provides a comprehensive range of services based on our matured platform, which involves in the custom antibody development service, from antigen design and epitope prediction to antibody purification and labeling.
Technology
Monoclonal antibody technology is based on immunization of animals with the interest antigen, for which mass-producing antibodies by cell line generated from the fusion of antibody-producing B-cells with a myeloma cell line. The new generated hybrid cells, sequentially are cloned to obtain stable monoclonal cell lines. The generation of functional, highly specific, high-affinity mAbs from monoclonal antibody development is a five-step process. Here we briefly list them as below:
|
Step 1: The development and optimization of an immunogenic antigen (Ag). Step 2: The host animal is immunized with the Ag to elicit an immune response and initiate the process of B-cell maturation. Step 3: The isolation of B cells from the host animal and fusion with myeloma cells to generate monoclonal antibody development. Step 4: The generated monoclonal antibody development are subject to screen and select. Step 5: The amplification of specific monoclonal antibody development and subsequent mAb purification. |
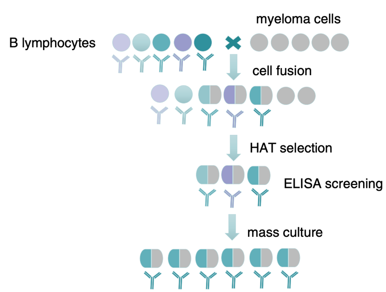 Fig.1 Process of monoclonal antibody generation technology.1
Fig.1 Process of monoclonal antibody generation technology.1
Creative Biolabs is an expert in the usage of monoclonal antibody development technology, a powerful method for antibody development. The mAbs produced have broad utility as therapeutics, diagnostic tools, and research reagents. The approach enables discovery of antibodies that bind to a variety of epitopes on the target molecule and, coupled with a robust screening platform, allows for identification of clones with the most desirable characteristics.
Benefits of Monoclonal Antibody Development Technology
- Natural affinity maturation
Natural affinity maturation (AM) is a complex antibody refinement process that occurs within the germinal centers of the host animal. monoclonal antibody development technology takes mature B cells from secondary lymphatic organs. The purified mAbs from these cells have already gone through stringent genetic modifications in the host animal.
- Low level of immunogenicity
The process of mAb generation from monoclonal antibody development takes advantage of natural complementary-determining regions (CDR) development. Thus, there is very limited risk of those CDRs causing an immune response.
- Constant domain activity
The constant domain of the antibody has to go through its own maturation process. Antibodies will go through class switch recombination (CSR) in order to change their lgM isotype. CSR can generate antibodies from all five isotypes in order to ensure that the antibody is equipped with the necessary effector functions and different bio-distribution.
Monoclonal Antibody Development Platform
Creative Biolabs offers the custom antibody design project based on our matured monoclonal antibody development platform to meet customer’ various needs. Experts at Creative Biolabs have the confidence to offer the satisfactory services and high-quality products through our monoclonal antibody development platform.
|
Immunogen Selection Immunization Monoclonal Antibody Development Fusion and Screening Sub-cloning of Selected Clones Expansion and Monoclonal Antibody Production |
Other services
As an industrial leader specialized in custom monoclonal antibody development services, Creative Biolabs also provides:
- Antigen design and epitope prediction
- Custom peptide synthesis, Protein production or DNA optimized immunization
- Affinity purification of final bleed
- Monoclonal antibody development sequencing
- Bioengineering and recombinant production
- Antibody production and purification from mouse monoclonal cell lines
Our monoclonal antibody development platform generates new expressing antibodies to your antigen of interest. If you have any question about monoclonal antibody development technology, do not hesitate to contact us now.
Reference
- Listek, M., et al. “A novel selection strategy for antibody producing hybridoma cells based on a new transgenic fusion cell line.” Scientific reports, 2020, 10: 1664. Distributed under Open Access license CC BY 4.0, without modification.